Personalized Vacuum Bell for Pectus Excavatum Treatment
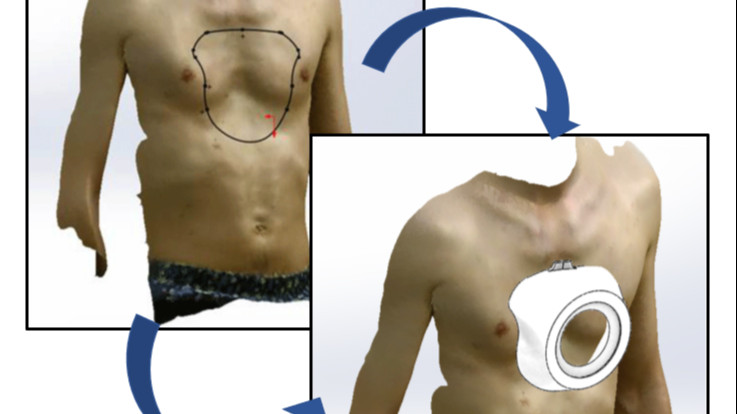
In this challenge, participants are tasked with creating a CAD design for a personalized Vacuum Bell, specifically tailored for the treatment of Pectus Excavatum. A 3D scan file (STL) of a patient will be provided as a reference. Alongside the CAD file for the Vacuum Bell, participants are required to furnish a detailed description of the chosen manufacturing technique.
Design Guidelines:
The Vacuum Bell design should be personalized to fit the anatomical specifications derived from the provided 3D scan. Participants must consider the comfort, effectiveness, and safety of the device in the treatment of Pectus Excavatum. The design should optimize contact with the patient's chest while ensuring ease of use and adherence to medical standards.
Required Specifications:
In addition to the CAD file, participants must articulate the selected manufacturing technique for bringing the Vacuum Bell to life. This includes detailing the materials chosen, the manufacturing processes involved, and any other pertinent considerations integral to the fabrication of the personalized medical device.
Author
No files
Precision and Fit (30 points):
- Accuracy and precision in adapting the Vacuum Bell to the patient's specific anatomical details.
- Demonstrated understanding of the medical requirements for treating Pectus Excavatum.
Effectiveness in Treatment (25 points):
- Consideration of medical efficacy in the design, ensuring it addresses the therapeutic needs of Pectus Excavatum.
- Potential for patient comfort and adherence to treatment protocols.
Innovative Design (20 points):
- Creativity in the overall CAD design, with emphasis on innovative solutions for personalized medical treatment.
- Integration of features that enhance the usability and effectiveness of the Vacuum Bell.
Feasibility for Manufacturing (15 points):
- Clarity and viability of the chosen manufacturing technique.
- Consideration of materials, scalability, and practical challenges associated with individual fabrication.
Documentation and Explanation (10 points):
- Clarity and completeness of the documentation accompanying the CAD design.
- Comprehensive explanation of the chosen manufacturing technique, detailing materials, processes, and adherence to medical standards.
Free registration to ASME MESA 2024 Conference